Regulatory T Cell Therapy in Turkey
Healthy Türkiye helps you find the best regulatory t cell in Turkey at affordable prices and adopts a 360-degree service approach in all areas of health through affiliated hospitals.
- Medical Treatment
- Cancer Treatment in Turkey
- Image Guided Radiotherapy in Turkey
- Image-Guided Radiosurgery in Turkey
- Intensity-Modulated Radiation Therapy in Turkey
- Kidney Cancer Treatment in Turkey
- Leukemia Treatment in Turkey
- Liver Cancer Treatment in Turkey
- Lung Cancer Treatment in Turkey
- Lymphoma Treatment in Turkey
- Bladder Cancer Treatment in Turkey
- Bone Marrow Transplant in Turkey
- Brachytherapy in Turkey
- Brain Cancer Treatment in Turkey
- Breast Cancer Treatment in Turkey
- Cervical Cancer Treatment in Turkey
- Chemotherapy in Turkey
- Colon Cancer Treatment in Turkey
- Hormonal Therapy in Turkey
- Bone Cancer Treatment in Turkey
- Endometrial Cancer Treatment in Turkey
- Gastric Cancer Treatment in Turkey
- Gene Therapy in Turkey
- Melanoma Treatment in Turkey
- Mesothelioma Treatment in Turkey
- Metastatic Cancer Treatment in Turkey
- Mouth Cancer Treatment in Turkey
- Neuroblastoma Treatment in Turkey
- Oral Cancer Treatment in Turkey
- Ovarian Cancer Treatment in Turkey
- Pancreatic Cancer Treatment in Turkey
- Photodynamic Therapy in Turkey
- Proton Therapy in Turkey
- Sarcoma Treatment in Turkey
- Skin Cancer Treatment in Turkey
- Stereotactic Body Radiation Therapy in Turkey
- Targeted Therapy in Turkey
- Testicular Cancer Treatment in Turkey
- Throat Cancer Treatment in Turkey
- Thyroid Cancer Treatment in Turkey
- Uterine Cancer Treatment in Turkey
- Volumetric-Modulated Arc Therapy in Turkey
- Prostate Cancer Treatment in Turkey
- Robotic Radical Prostatectomy Surgery in Turkey
- Hemato-Oncology Treatment in Turkey
- Skin Cancer Malignant Melanoma Treatment in Turkey
- Colorectal Cancer Treatment in Turkey
- Radiotherapy in Turkey
- Immunotherapy in Turkey
- TIL Therapy in Turkey
- TCR-T Therapy in Turkey
- CAR NK Cell Therapy in Turkey
- Gene Editing Therapy in Turkey
- Dendritic Cell Vaccines in Turkey
- Oncolytic Virus Therapy in Turkey
- NK-92 Cell Therapy in Turkey
- Cytokine Therapy in Turkey
- Bispecific T-cell Engager in Turkey
- Macrophage-Based Therapy in Turkey
- IPSC-Derived Immunotherapies in Turkey
- Glossectomy in Turkey
- Regulatory T Cell Therapy in Turkey
- Gamma Delta T Cells Therapy in Turkey
- Homepage
- Medical Treatment
- Regulatory T Cell Therapy in Turkey

Regulatory T Cell (Treg) Therapy in Turkey
Regulatory T cell (Treg) therapy in Turkey is an emerging cutting-edge treatment aimed at restoring immune balance for patients with serious autoimmune and inflammatory conditions. Tregs are often called the immune system’s “peacekeepers” – specialized cells that prevent the immune system from attacking the body’s own tissues. Turkey’s advanced hospitals and research centers are among the early adopters of Treg therapy, providing access to this experimental but promising treatment under expert supervision and strict safety standards.
Overall, regulatory T cell therapy represents a new frontier in precision immunotherapy – modulating the immune system with its own cells – and Turkey is among the countries at the forefront of translating this science into clinical care.

Treg Therapy in Turkey
Regulatory T cell therapy Turkey is a form of adoptive cellular immunotherapy that uses the patient’s own immune cells (or donor cells) to regulate harmful immune reactions. Tregs are a subset of T-lymphocytes whose natural job is to suppress excessive immune responses and maintain tolerance to self-antigens. In autoimmune diseases, the body’s immune system mistakenly attacks its own organs; Tregs normally prevent this, but patients often have dysfunctional or insufficient Tregs. The idea behind Treg therapy is to boost the number or activity of these “security guard” cells and reinfuse them into the patient to calm down abnormal immunity.
In practice, Treg therapy Turkey involves isolating Treg cells from the blood, expanding or enhancing them in a laboratory, and then infusing them back into the patient. Once infused, the regulatory T cells travel through the body and dampen inappropriate immune attacks – for example, by secreting anti-inflammatory cytokines and directly interacting with other immune cells to suppress their activity. This can help halt the progression of autoimmune damage and restore a healthier immune balance without the broad side effects of general immunosuppressive drugs.
We Care About Your Health
Healthy Türkiye provides the best for your health and comfort. You will feel privileged with us.
7/24 Quality Personal Assistance Throughout Your Journey
Customizable for You All-Inclusive Packages
Get the Right Advice for your Health
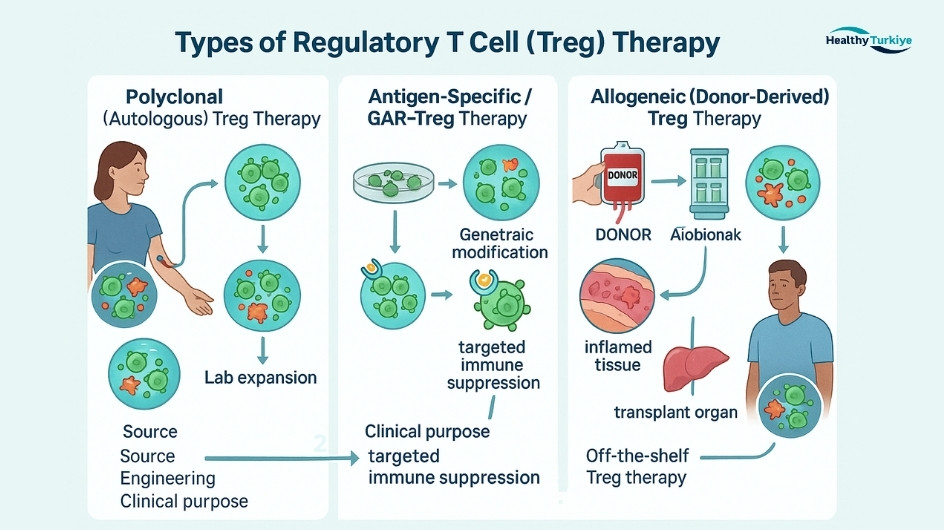
Types of Regulatory T Cell Therapy
There are several approaches to Treg therapy, reflecting how the cells are sourced and engineered. The main types of Treg therapy include:
Polyclonal Treg Therapy (Autologous)
This is the simplest form, using the patient’s own regulatory T cells expanded in the lab. “Polyclonal” means the Tregs are not targeted to any single antigen – they have broad suppressive activity. Early trials using polyclonal autologous Tregs (for example, in graft-versus-host disease and Crohn’s disease) showed encouraging safety and some symptom improvement. However, outcomes in diseases like type 1 diabetes indicated that polyclonal Tregs had limited efficacy in halting autoimmunity when given in small numbers. One challenge is obtaining enough stable Tregs, since they are rare in blood and can lose their regulatory phenotype during expansion.
Researchers are working on solutions – for instance, genetic tweaks to lock in the Treg identity and make them function more powerfully. Autologous Treg therapy has the advantage of using the patient’s own cells (no rejection risk), but it may require lengthy cell culture times and the suppressive effect is nonspecific.
Antigen-Specific Treg Therapy
To increase effectiveness, scientists are developing Tregs that target specific proteins or tissues involved in a patient’s disease. These antigen-specific Tregs can be generated by selective expansion of Tregs that recognize a particular autoantigen, or by genetic engineering. Studies have found that antigen-specific Tregs are more potent than polyclonal Tregs in suppressing disease-causing immune responses.
A cutting-edge example is CAR-Treg therapy, where Tregs are genetically modified with a Chimeric Antigen Receptor (CAR) or engineered T-cell receptor to home in on a target (similar to CAR-T cells in cancer, but here used to suppress immunity rather than attack tumors). CAR-Treg technology is being explored for conditions like organ transplantation – e.g. creating Tregs that recognize an HLA molecule of a transplanted organ to protect it from rejection. While CAR-Tregs are still in early-phase trials, they hold promise due to their high specificity and enhanced suppressive functions. The manufacturing process for CAR-Tregs is complex (even more than cancer CAR-T cells) because it requires isolating rare Tregs and genetically modifying them, but if successful, CAR-Tregs could provide targeted control of autoimmunity with long-lasting effects.
Allogeneic (Donor-Derived) Treg Therapy
In some cases, Tregs can come from a donor rather than the patient. For example, Tregs can be isolated from donated umbilical cord blood or from a healthy donor’s peripheral blood. These donor Tregs can be expanded and given “off-the-shelf.” Notably, using donor Tregs is quite different from using donor conventional T cells, because Tregs by nature suppress immune reactions rather than cause them – meaning they are unlikely to provoke graft-versus-host disease. In fact, a landmark 2009 study in bone marrow transplant patients showed that donor-derived Tregs could be infused to reduce graft-versus-host disease and the need for immunosuppressant drugs. Similarly, donor Tregs were tested in children with refractory GVHD and led to clinical improvements.
Allogeneic Treg products, including those derived from stem cells or induced pluripotent stem cells, are under development to create a readily available “off-the-shelf” Treg therapy. The appeal is that this could eliminate the delay of growing a patient’s own cells. Turkey’s cell therapy centers are interested in these advances – having access to cell banks and GMP manufacturing means international patients might receive donor Treg infusions without the wait if they qualify for an applicable program.
In summary, Treg therapies can be customized in various ways: autologous vs. donor source, unmodified vs. CAR-engineered, broad vs. antigen-specific. The field is rapidly evolving. Turkish clinics stay up-to-date with these developments, often in collaboration with global research. Depending on each patient’s condition and the protocols available, the treatment team will decide which type of Treg therapy is most appropriate or available – ensuring the highest chance of success with safety.
Who Is a Candidate for Regulatory T Cell (Treg) Therapy in Turkey
Regulatory T Cell (Treg) therapy in Turkey is designed for patients with autoimmune, inflammatory, or transplant-related conditions that are severe or resistant to standard treatments. It aims to rebalance the immune system by increasing the number of regulatory T cells that control inflammation and immune overactivity.
Autoimmune Diseases: Treg therapy may help patients with autoimmune disorders such as type 1 diabetes, rheumatoid arthritis, lupus (SLE), multiple sclerosis (MS), and inflammatory bowel diseases like Crohn’s or ulcerative colitis. These conditions occur when the immune system attacks the body’s tissues. Increasing Treg cells helps reduce inflammation and prevent further damage. Candidates typically have moderate to severe disease that hasn’t responded well to medications like steroids, biologics, or immunosuppressants.
Organ or Bone Marrow Transplant Patients: Patients who have undergone organ transplants (kidney, liver, etc.) or bone marrow transplants may also qualify. Treg therapy can reduce rejection and decrease the need for lifelong immunosuppressant drugs. In transplant cases, infusing Tregs helps train the immune system to accept the new organ safely and reduce graft-versus-host disease (GVHD). Turkish transplant centers are conducting studies to make this approach more widely available.
Other Immune-Mediated or Inflammatory Conditions: Treg therapy may also support patients with allergies, asthma, or rare immune dysregulation syndromes. Research shows benefits in early-stage type 1 diabetes, helping preserve pancreatic function and slow disease progression. Candidates with chronic inflammation not managed by standard treatments can also be evaluated.
General Health Requirements: Patients should be in overall good health aside from their immune condition. Treg therapy isn’t suitable for those with active infections or cancer, as it could suppress the body’s ability to fight those illnesses. Before treatment, patients undergo a full medical evaluation to ensure safety.
Understanding and Access: Because Treg therapy is still experimental, ideal candidates are those open to participating in clinical or institutional programs. International patients traveling to Turkey often work with Healthy Türkiye or its partner clinics to review their medical records and confirm eligibility.
In short, Treg therapy in Turkey offers new hope for patients with difficult-to-treat immune diseases or transplant complications, providing a potential “immune reset” where conventional treatments have failed.
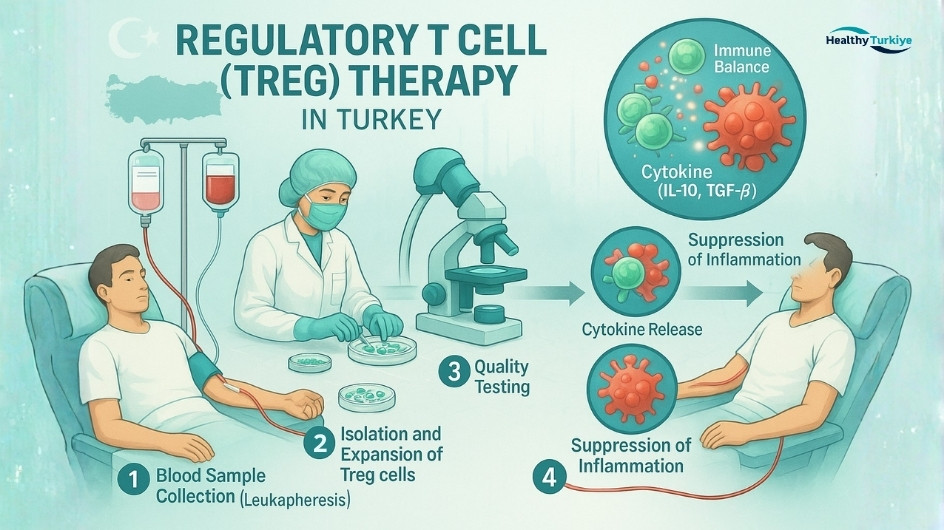
How is Regulatory T Cell (Treg) Therapy performed in Turkey?
Regulatory T cell therapy in Turkey follows a clear, GMP-guided pathway designed for safety and consistency.
Initial consultation and evaluation: Multidisciplinary review (immunology/hematology), medical history, labs and imaging to confirm eligibility; medications adjusted if needed.
Cell collection: White blood cells are collected in an outpatient session to obtain autologous Tregs; donor or cord-blood Tregs may be sourced when protocols allow.
Laboratory processing and expansion (GMP): Tregs are isolated (e.g., CD4+CD25+CD127low), expanded 1–2 weeks with IL-2/activation signals; optional antigen-specific or engineered (CAR-Treg) workflows in approved programs.
Quality control and preparation: Sterility, viability, phenotype, and potency testing; some protocols use mild conditioning or low-dose IL-2 to support engraftment.
Treg infusion: Intravenous infusion in a monitored clinic setting; typically painless, 30–60 minutes, with short observation afterward.
Post-treatment follow-up: Short in-country monitoring, discharge with a detailed report, and telemedicine check-ins; ongoing labs track response and safety.
Timing and stay: From collection to infusion usually takes a few weeks (driven by lab expansion). Many international patients plan a 2–3-week stay or two short visits.
Non-invasive workflow, rigorous GMP quality controls, low rate of serious adverse events, and coordinated care tailored to international patients seeking regulatory T cell therapy in Turkey.
Benefits of Regulatory T Cell (Treg) Therapy
Regulatory T cell therapy provides significant advantages for patients with autoimmune and inflammatory diseases, offering a precise and safer approach compared to conventional treatments.
Targets the root cause of autoimmunity:
Instead of broadly suppressing the immune system like steroids or immunosuppressants, Treg therapy restores natural immune balance. It reprograms immune cells to stop attacking the body, helping control diseases such as type 1 diabetes, lupus, and multiple sclerosis at their source.
Reduces dependency on long-term medication:
Successful Treg therapy may minimize or replace the need for daily immunosuppressant drugs, which often cause side effects like infection or organ damage. This means fewer medications, fewer side effects, and better quality of life for patients and transplant recipients.
High safety and tolerability:
Treg therapy uses the body’s own cells, reducing risks of rejection or severe reactions. Clinical studies show no serious side effects, with only mild temporary symptoms in rare cases. This makes it suitable for older or medically fragile patients.
Potential for long-term remission:
By “resetting” the immune system, Treg therapy can lead to sustained remission after just one or two infusions. Trials have shown stabilized disease markers and long-lasting improvements, suggesting a durable immune balance.
Personalized and flexible treatment:
Each Treg therapy is tailored to the patient. Doses can be adjusted, and repeat treatments can safely reinforce benefits. This precision medicine approach ensures a customized plan for optimal results.
Access to cutting-edge innovation in Turkey:
Turkish hospitals combine global research with advanced cell labs, offering patients early access to the latest protocols and safe, cost-effective treatment.
In summary, Treg therapy provides a natural, targeted, and durable solution for immune disorders — and Turkey’s expertise makes it one of the most promising destinations for this next-generation therapy.

2026 Cost of Regulatory T Cell Therapy in Turkey
All types of medical attentions like regulatory t cell therapy are very affordable in Turkey. Many factors are also included in determining the cost of regulatory t cell therapy in Turkey. Your process with Healthy Türkiye will last from the time you decide to have a treg in Turkey until the time you are fully recovered even if you are back home. The exact regulatory t cell therapy procedure cost in Turkey depends on the type of operation involved.
The cost of regulatory t cell therapy in Turkey does not demonstrate many variations in 2026. Compared to costs in developed countries like the United States or the UK, regulatory t cell therapy costs in Turkey are relatively low. So, it's no wonder patients from across the world visit Turkey for regulatory t cell therapy procedures. However, the price is not the only factor affecting choices. We suggest looking for hospitals that are safe and have regulatory t cell therapy reviews on Google. When people decide to seek medical help for regulatory t cell therapy, they will not only have had low-cost procedures in Turkey, but also the safest and best treatment.
At clinics or hospitals contracted with Healthy Türkiye, patients will receive the best treg from specialist doctors in Turkey at affordable rates. Healthy Türkiye teams provide medical attention regulatory t cell therapy procedures and high-quality treatment to patients at a minimum cost. When you contact Healthy Türkiye assistants, you can get free information about the cost of regulatory t cell therapy in Turkey and what this cost covers.
Why Is Regulatory T Cell Therapy Cheaper in Turkey?
One of the main considerations before traveling abroad for regulatory t cell therapy is the cost-effectiveness of the whole process. Many patients think that when they add flight tickets and hotel expenses to their treg costs, it will become very expensive to travel, which is not true. Contrary to popular belief, round-trip flight tickets to Turkey for regulatory t cell therapy can be booked very affordably.
In this case, assuming you are staying in Turkey for your regulatory t cell therapy, your total travel expense of flight tickets and accommodation will only cost less than any other developed country, which is nothing compared to the amount that you are saving.
The question “why regulatory t cell therapy cheaper in Turkey?” is so common between patients or people simply curious about getting their medical treatment in Turkey. When it comes to regulatory t cell therapy prices in Turkey, there are 3 factors allowing cheaper prices:
The currency exchange is favorable for whoever looking for regulatory t cell therapy has a euro, dollar, or pound;
The lower cost of living and cheaper overall medical expenses such as regulatory t cell therapy;
For regulatory t cell therapy, incentives are given by the Turkish Government to medical clinics working with international clients;
All these factors allow for cheaper regulatory t cell therapy prices, but let’s be clear, these prices are cheaper for people with strong currencies (as we said, euro, dollar, Canadian dollar, pound, etc).
Every year, thousands of patients from all over the world come to Turkey to get treg. The success of the healthcare system has increased in recent years, especially for regulatory t cell therapy. It’s easy to find well-educated and English-speaking medical professionals in Turkey for all kinds of medical treatment such as regulatory t cell therapy.

Why Choose Turkey for Regulatory T Cell Therapy?
Turkey is a common choice among international patients seeking advanced regulatory t cell therapy. Turkey’s health procedures are safe and effective operations with a high success rate like regulatory t cell therapy. The increasing demand for high-quality treg at affordable prices has made Turkey a popular medical travel destination. In Turkey, regulatory t cell therapy is performed by highly experienced and trained doctors with the most advanced technology in the world. regulatory t cell therapy is done in Istanbul, Ankara, Antalya, and other major cities. The reasons for choosing regulatory t cell therapy in Turkey are as follows:
High-quality hospitals: Joint Commission International (JCI) accredited hospitals have dedicated treg units that are specially designed for patients. International and national strict protocols provide effective and successful regulatory t cell therapy for patients in Turkey.
Qualified experts: The expert teams include nurses and specialist doctors, together to carry out regulatory t cell therapy according to the patient's needs. All the included doctors are highly experienced in performing regulatory t cell therapy.
Affordable price: The cost of regulatory t cell therapy in Turkey is affordable compared to Europe, the USA, the UK, Singapore, Australia, etc.
The high success rate: Highly experienced specialists, the best available technology, and stringently followed safety guidelines for post-operative care of the patient, resulting in a high success rate for regulatory t cell therapy in Turkey.
Is Regulatory T Cell Therapy Safe in Turkey?
Did you know Turkey is one of the most visited destinations for regulatory t cell therapy in the world? It is ranked one of the most visited tourist destinations for regulatory t cell therapy. Over the years it has also come to be a very popular medical tourism destination too with many tourists coming in for treg. There are so many reasons why Turkey stands out as a leading destination for regulatory t cell therapy. Because Turkey is both safe and easy to travel to too with a regional airport hub and flight connections to pretty much everywhere, it is preferred for regulatory t cell therapy.
The best hospitals in Turkey have experienced medical staff and specialists who have performed thousands of medical services such as regulatory t cell therapy. All procedures and coordination related to regulatory t cell therapy are controlled by the Ministry of Health in accordance with the law. Over many years, the greatest progress in medicine has been observed in the field of treg. Turkey is known among foreign patients for its great opportunities in the area of regulatory t cell therapy.
To emphasize, besides the price itself, the key factor in selecting a destination for regulatory t cell therapy is certainly the standard of medical services, the hospital staff’s high expertise, hospitality, and the safety of the country.
All-Inclusive Package for Regulatory T Cell Therapy in Turkey
Healthy Türkiye offers all-inclusive packages for regulatory t cell therapy in Turkey at much lower prices. Extremely professional and experienced doctors and technicians carry out the high-quality regulatory t cell therapy. The cost of regulatory t cell therapy in European countries can be quite expensive, especially in the UK. Healthy Türkiye provides cheap all-inclusive packages for a long and short stay of regulatory t cell therapy in Turkey. Because of many factors, we can provide you with many opportunities for your treg in Turkey.
The price of regulatory t cell therapy differs from other countries due to medical fees, staff labor prices, exchange rates, and market competition. You can save much more in regulatory t cell therapy compared to other countries in Turkey. When you purchase regulatory t cell therapy all-inclusive package with Healthy Türkiye our healthcare team will present of hotels for you to choose from. In regulatory t cell therapy travel, you will have the price of your stay included in the all-inclusive package cost.
In Turkey, when you purchase regulatory t cell therapy all-inclusive packages through Healthy Türkiye, you will always receive VIP transfers. These are provided by Healthy Türkiye, which is contracted with highly qualified hospitals for treg in Turkey. Healthy Türkiye teams will organize everything about regulatory t cell therapy for you and have you picked up from the airport and safely brought to your accommodation. Once settled in the hotel, you will be transferred to and from the clinic or hospital for regulatory t cell therapy. After your regulatory t cell therapy has been successfully completed, the transfer team will return you to the airport in time for your flight home. In Turkey, all packages of regulatory t cell therapy can be arranged upon request, which relaxes the minds of our patients. You can reach out to Healthy Türkiye for everything you need to know about regulatory t cell therapy in Turkey.
Frequently Asked Questions
While stem cell therapy regenerates damaged tissues, Treg therapy specifically targets the immune system, restoring tolerance and reducing autoimmune attacks.
Improvements may appear within weeks to months, depending on the condition and immune response, with effects lasting long-term in many cases.
Yes, it’s often used alongside low-dose immunosuppressants or IL-2 therapy to enhance immune balance and maintain results safely.
Currently, Treg therapy is under clinical investigation worldwide. Many studies have shown safety and potential efficacy, but large-scale approval is ongoing.
Most patients require one or two infusions, though some may benefit from periodic maintenance treatments depending on their condition.
It cannot restore destroyed tissues but can stop further damage by reprogramming immune behavior, helping preserve remaining healthy cells.
