NK-92 Cell Therapy in Turkey
Healthy Türkiye helps you find the best NK-92 cell therapy in Turkey at affordable prices and adopts a 360-degree service approach in all areas of health through affiliated hospitals.
- Medical Treatment
- Cancer Treatment in Turkey
- Image Guided Radiotherapy in Turkey
- Image-Guided Radiosurgery in Turkey
- Intensity-Modulated Radiation Therapy in Turkey
- Kidney Cancer Treatment in Turkey
- Leukemia Treatment in Turkey
- Liver Cancer Treatment in Turkey
- Lung Cancer Treatment in Turkey
- Lymphoma Treatment in Turkey
- Bladder Cancer Treatment in Turkey
- Bone Marrow Transplant in Turkey
- Brachytherapy in Turkey
- Brain Cancer Treatment in Turkey
- Breast Cancer Treatment in Turkey
- Cervical Cancer Treatment in Turkey
- Chemotherapy in Turkey
- Colon Cancer Treatment in Turkey
- Hormonal Therapy in Turkey
- Bone Cancer Treatment in Turkey
- Endometrial Cancer Treatment in Turkey
- Gastric Cancer Treatment in Turkey
- Gene Therapy in Turkey
- Melanoma Treatment in Turkey
- Mesothelioma Treatment in Turkey
- Metastatic Cancer Treatment in Turkey
- Mouth Cancer Treatment in Turkey
- Neuroblastoma Treatment in Turkey
- Oral Cancer Treatment in Turkey
- Ovarian Cancer Treatment in Turkey
- Pancreatic Cancer Treatment in Turkey
- Photodynamic Therapy in Turkey
- Proton Therapy in Turkey
- Sarcoma Treatment in Turkey
- Skin Cancer Treatment in Turkey
- Stereotactic Body Radiation Therapy in Turkey
- Targeted Therapy in Turkey
- Testicular Cancer Treatment in Turkey
- Throat Cancer Treatment in Turkey
- Thyroid Cancer Treatment in Turkey
- Uterine Cancer Treatment in Turkey
- Volumetric-Modulated Arc Therapy in Turkey
- Prostate Cancer Treatment in Turkey
- Robotic Radical Prostatectomy Surgery in Turkey
- Hemato-Oncology Treatment in Turkey
- Skin Cancer Malignant Melanoma Treatment in Turkey
- Colorectal Cancer Treatment in Turkey
- Radiotherapy in Turkey
- Immunotherapy in Turkey
- TIL Therapy in Turkey
- TCR-T Therapy in Turkey
- CAR NK Cell Therapy in Turkey
- Gene Editing Therapy in Turkey
- Dendritic Cell Vaccines in Turkey
- Oncolytic Virus Therapy in Turkey
- NK-92 Cell Therapy in Turkey
- Cytokine Therapy in Turkey
- Bispecific T-cell Engager in Turkey
- Macrophage-Based Therapy in Turkey
- IPSC-Derived Immunotherapies in Turkey
- Glossectomy in Turkey
- Regulatory T Cell Therapy in Turkey
- Gamma Delta T Cells Therapy in Turkey
- Homepage
- Medical Treatment
- NK-92 Cell Therapy in Turkey
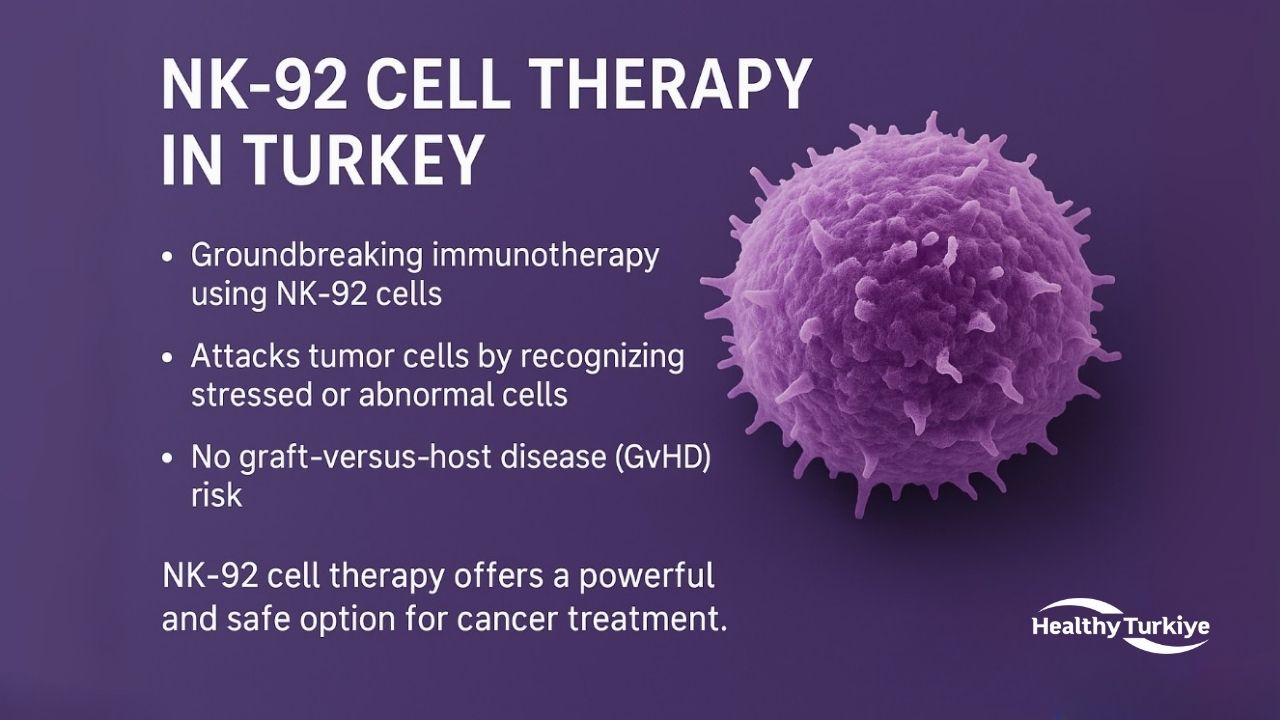
NK 92 Cell Therapy Turkey
NK-92 cell therapy in Turkey is a groundbreaking immunotherapy that offers a revolutionary approach to cancer treatment. NK-92 cells are a permanent, IL-2-dependent NK cell line derived from a lymphoma patient, allowing them to be produced in large batches in GMP-compliant cell banks without the need for harvesting cells from each individual patient. NK 92 cell therapy in Turkey works similarly to normal NK cells, attacking tumor cells by recognizing stressed or abnormal cells and releasing perforin/granzyme to kill them. An important feature of this therapy is that it does not cause graft-versus-host disease (GvHD), making it suitable for any patient without the need for matching.
NK-92 cell therapy in Turkey provides a powerful and safe option for cancer treatment. The therapy induces a strong immune response against cancer cells, without causing severe side effects for the patient. NK-92 cells recognize and target tumor cells by detecting abnormal or stressed cells, subsequently eliminating them. For those considering NK-92 cell therapy in Turkey, Healthy Türkiye can provide expert guidance and support throughout the process.
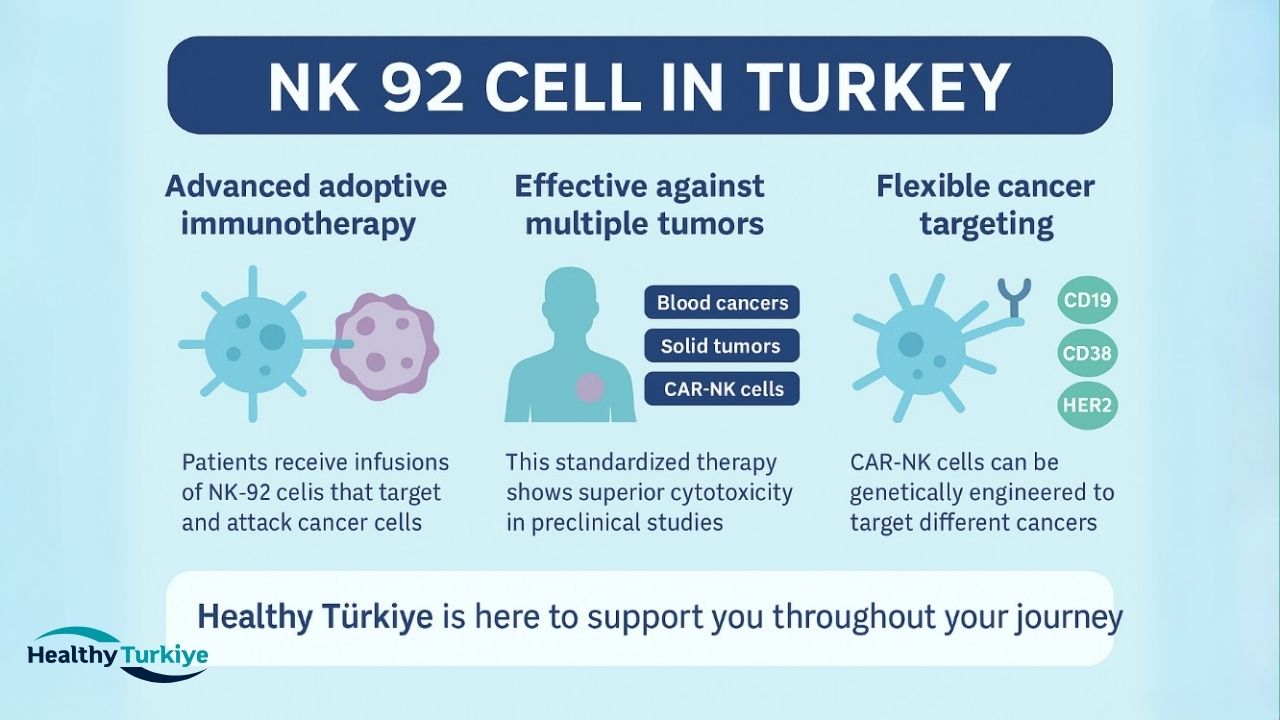
NK 92 Cell in Turkey
NK 92 cell therapy in Turkey is an advanced form of adoptive immunotherapy, where patients receive live NK-92 cell infusions that circulate in the body, targeting and attacking cancer cells. In laboratory settings, NK-92 cells are often genetically engineered, such as with chimeric antigen receptors (CARs), to better target specific tumors. In blood cancers, NK-92 Cell Therapy in Turkey can directly eliminate cancer cells or use antibody-dependent mechanisms.
For solid tumors, these cells can infiltrate and destroy tumor cells locally, or enhance the effectiveness of other therapies. Thanks to NK-92's standardized nature, each infusion maintains consistent activity, with preclinical studies showing its superior cytotoxicity against multiple tumor types. High-dose infusions have proven safe, with no signs of toxicity or "engraftment" in animal models, indicating that the cells are effective without causing long-term harm.
NK-92 Cell in Turkey also offers the flexibility of modifying NK-92 cells into CAR-NK cells, enhancing their ability to target a wide range of cancers. Examples include CARs targeting CD19/CD20 for B-cell leukemias and lymphomas, CD38 for myeloma, and HER2 for breast and epithelial cancers. These genetic modifications expand the variety of tumors that NK-92 Cell in Turkey can recognize and destroy, making it a promising treatment for many cancer types. For personalized support and treatment solutions in Turkey, Healthy Türkiye is ready to help you throughout your journey.
Why Consider NK-92 Therapy?
NK-92 cell therapy in Turkey offers a unique and promising solution for cancer treatment, providing multiple advantages for patients. This therapy is gaining attention due to its "off-the-shelf" availability, high consistency, and excellent safety profile. Here are the key reasons why NK-92 therapy stands out:
“Off-the-shelf” availability: NK-92 cells are sourced from a frozen master cell bank and can be expanded in vitro, eliminating the need for patient-specific cell harvesting. This ensures faster treatment without delays or variability often seen in patient-derived therapies.
No GvHD risk: Unlike other therapies, NK-92 cell therapy in Turkey does not cause graft-versus-host disease (GvHD), as the NK cells do not attack normal host tissues. This means that any patient, regardless of HLA compatibility, can benefit from this therapy without the need for donor matching.
Consistency and potency: All NK-92 doses come from the same standardized cell line, ensuring consistent and reliable therapeutic effects. The cells have a natural ability to kill tumor cells through innate mechanisms and can be further enhanced with CARs (Chimeric Antigen Receptors) for more targeted actions.
Flexibility: Researchers have developed NK-92 variants like NK-92MI, which secrete IL-2, enabling patients to avoid high-dose IL-2 infusions. This helps to reduce toxicity commonly associated with systemic IL-2 treatments.
Safety profile: Published trials show that NK-92 cell therapy in Turkey is generally very well-tolerated, even with high doses of up to billions of cells per infusion. Side effects are typically mild and transient, without the severe cytokine release reactions seen in other therapies like CAR-T.
NK 92 cell therapy in Turkey provides a safer, more accessible option for patients seeking advanced cancer treatment. Its standardized production and flexibility make it a highly effective therapy with broad applications, offering hope to those in need of alternative cancer treatments.
We Care About Your Health
Healthy Türkiye provides the best for your health and comfort. You will feel privileged with us.
7/24 Quality Personal Assistance Throughout Your Journey
Customizable for You All-Inclusive Packages
Get the Right Advice for your Health
Types of Cancer Treated with NK-92 Cell Therapy
NK-92 Cell Therapy in Turkey is being explored as a promising treatment for various types of cancer, offering new hope for patients with hard-to-treat tumors. This therapy targets specific tumor characteristics, such as low MHC or high stress ligands, making it an effective option for different cancer types. Here's how NK-92 is being applied across various cancers:
Hematologic malignancies: Clinical trials have shown positive results for relapsed and refractory lymphoma and myeloma patients treated with NK-92 cell therapy in Turkey. A Phase I study in Canada demonstrated complete remissions in two heavily pretreated lymphoma/myeloma patients, while trials for acute myeloid leukemia (AML) and acute lymphoblastic leukemia are ongoing, often utilizing CAR-NK-92 cells.
Lung cancer: Preclinical studies and early human trials suggest that NK-92 Cell Therapy in Turkey can effectively target lung tumors. One review highlighted that NK-92 infusions in lung cancer patients achieved on-target tumor killing with no off-target effects, and trials targeting lung metastases are underway.
Solid tumors: NK-92 cell therapy in Turkey has been injected into various refractory tumors. A case report from Turkey demonstrated its effectiveness in treating a child’s relapsed Ewing sarcoma, showing partial tumor regression without toxicity. Additional research is testing NK-92 Cell in Turkey for breast cancer, glioblastoma, and pancreatic cancer using CAR-armed NK-92 cells.
Engineered targets: Researchers are further enhancing the power of NK-92 cell therapy in Turkey by arming NK-92 with CARs. For example, CAR-NK-92 targeting CD19/CD22 is used against B-cell leukemias, and HER2-CAR NK-92 targets HER2-positive breast cancer, expanding the range of cancers this therapy can address.
NK-92 cell therapy in Turkey offers a versatile and powerful approach for targeting a wide variety of cancers. Its ability to treat hematologic, lung, solid tumors, and engineered targets demonstrates its potential as a treatment option for many patients seeking innovative cancer therapies.
Who Is a Candidate for NK-92 Cell Therapy?
NK-92 cell therapy in Turkey is an experimental treatment, and eligibility for this therapy is typically tied to clinical trials or special protocols. It is generally recommended for patients who meet specific criteria, ensuring they are well-suited to benefit from the therapy's potential. Here's a breakdown of typical candidates:
Relapsed or refractory cancer: Patients who have exhausted standard treatments (chemotherapy, radiation, surgery, or approved immunotherapies) and whose cancer is progressing are prime candidates for NK-92 Cell Therapy in Turkey. Trials usually focus on aggressive or recurrent diseases.
Good performance status: Candidates must generally be in good physical condition (e.g., ECOG 0–2), allowing them to tolerate cell infusions. For instance, a trial for acute myeloid leukemia (AML) required patients to be ≥18 years old and have an ECOG score of ≤2.
Adequate organ function: Patients must have functioning organs, with lab results within safe limits. For example, trials required a creatinine level <2× normal, AST/ALT <5× normal, normal bilirubin, and LVEF ≥45%, ensuring safe therapy administration.
Disease measurable: There must be evidence of active disease (e.g., tumor detected on imaging or leukemia blasts) to assess the treatment's effectiveness.
Informed consent: Since NK-92 Cell Therapy in Turkey is not FDA-approved, patients must fully understand the risks and provide informed consent. Enrollment in a clinical trial or expanded-access program, along with ethical approval, is required.
In practice, anyone who meets these criteria and is willing to participate in an experimental treatment can consult with specialists to determine if NK-92 Cell Therapy in Turkey is a suitable option. Age limits are typically dictated by trial protocols, and some studies include children, as demonstrated in the Ewing sarcoma case.
Before NK-92 Cell Therapy in Turkey
Before starting NK-92 cell therapy in Turkey, it is important for patients to ensure they are well-hydrated and maintain proper nutrition to support their overall health. Patients may be advised to temporarily stop any immunosuppressive or myelosuppressive medications, as recommended by their doctor. Informed consent is a crucial part of the process, as patients and their guardians need to fully understand the experimental nature of the treatment and its associated risks. While NK-92 therapy does not require specific preparations like those for transplants, patients may receive additional vaccines, such as the seasonal flu vaccine, to optimize their immune system's health before treatment begins.
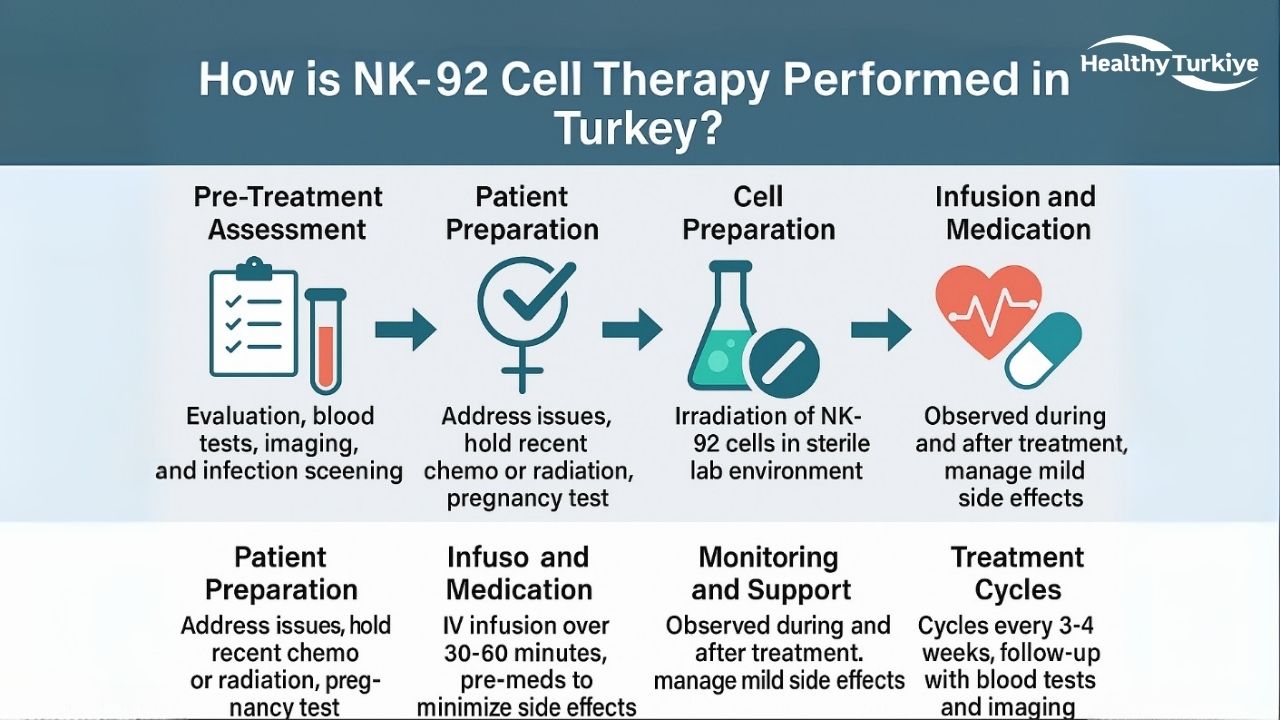
How is NK-92 Cell Therapy Performed in Turkey?
NK-92 cell therapy in Turkey begins with thorough preparatory steps to ensure the patient’s readiness for treatment. Before the therapy, patients undergo a comprehensive evaluation, including blood tests (CBC, kidney/liver panels, infection screening), heart function assessments (such as an echo), and imaging. Any active infections or uncontrolled illnesses must be addressed before proceeding. Recent chemotherapy or radiation treatments are usually paused for 1-2 weeks to avoid overlapping toxicities. Women of childbearing potential are required to have a negative pregnancy test and must use contraception during treatment. Informed consent is obtained to ensure that the patient fully understands the experimental nature of the nk 92 cell in Turkey and the associated risks.
The nk 92 cell therapy in Turkey procedure involves the infusion of NK-92 cells, which are prepared in a sterile laboratory. These cells are typically irradiated to prevent proliferation before being diluted to the appropriate dose. The infusion is administered intravenously over 30–60 minutes, with doses ranging from 1×10^9 to 5×10^9 cells per square meter of body surface. Standard pre-medications, such as acetaminophen and antihistamines, are given to minimize side effects like fever or chills. During and after the infusion, vital signs are monitored closely. Any mild side effects, such as fever or fatigue, are managed with supportive care. If the treatment is well-tolerated, it is usually repeated in cycles, typically every 3–4 weeks, with follow-up cycles lasting from 2–6 cycles, depending on the patient’s condition. Periodic monitoring with blood tests and imaging ensures that tumor response is being tracked effectively.
After NK-92 Cell Therapy in Turkey
After receiving NK-92 Cell Therapy in Turkey, post-infusion care is generally minimal. Most patients experience mild flu-like symptoms, such as fever and fatigue, which can be managed with acetaminophen for a day. It is important to stay well-hydrated and rest for the remainder of the day. There are no special infection precautions required, as NK-92 Cell Therapy in Turkey does not lead to lasting immune suppression. Regular follow-up with the medical team is essential, including blood tests to monitor organ function and tumor response through imaging. If symptoms persist beyond 24 hours, patients should contact their physician. In clinical trials, no prolonged adverse effects or long-term immunosuppression were reported, making aftercare focused mainly on monitoring for unexpected symptoms and providing ongoing medical support.
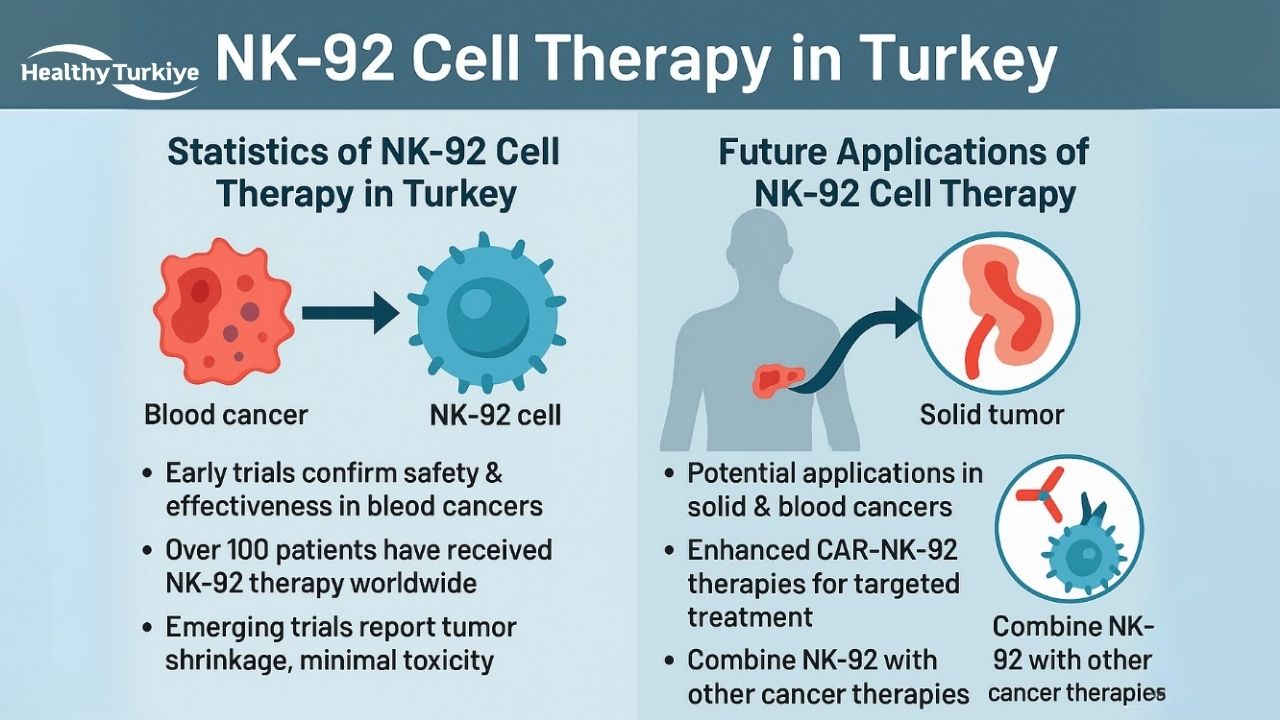
Statistics of NK-92 Cell Therapy in Turkey
NK-92 cell therapy in Turkey remains in the experimental phase, but early clinical trials and case reports have shown promising results. In a Phase I study conducted in Toronto, 12 adults with relapsed blood cancers (lymphoma/myeloma) received high-dose irradiated NK-92 infusions. Remarkably, two patients achieved complete remission, and others showed partial or minor responses, confirming the treatment’s safety and effectiveness. Additionally, a 2020 case report from Turkey demonstrated that NK-92 therapy, when injected into a child’s tumor, led to visible tumor shrinkage with no toxicity. Worldwide, over 100 patients have received NK-92 therapy, with early trials reporting minimal toxicity. Emerging trials are also exploring CAR-NK-92 for various cancers, with promising early data showing tumor shrinkage and no significant side effects.
Future Applications of NK-92 Cell Therapy
NK-92 Cell Therapy in Turkey holds immense potential for future cancer treatments, with ongoing research focused on expanding its applications across various malignancies. As researchers continue to explore the capabilities of NK-92 cells, their use is being adapted for more complex cancers, including solid tumors like glioblastoma and pancreatic cancer, as well as blood cancers such as acute myeloid leukemia and B-cell lymphomas. Future advancements may include enhanced CAR-NK-92 therapies, which can target specific tumor markers for more personalized and effective treatments. Furthermore, NK-92 therapy is being investigated for its potential use in combination with other immunotherapies, chemotherapy, and radiotherapy to create more comprehensive and potent treatment regimens. As research progresses, NK-92 therapy in Turkey may emerge as a key option in the fight against cancer, offering new hope for patients with few other treatment options.

2026 Cost of NK-92 Cell Therapy in Turkey
All types of medical attention, like NK-92 cell therapy, are very affordable in Turkey. Many factors are also included in determining the cost of NK-92 cell therapy in Turkey. Your process with Healthy Türkiye will last from the time you decide to have an NK-92 cell therapy in Turkey until the time you are fully recovered, even if you are back home. The exact NK-92 cell therapy procedure cost in Turkey depends on the type of operation involved.
The cost of NK-92 cell therapy in Turkey does not demonstrate many variations in 2026. Compared to costs in developed countries like the United States or the UK, NK-92 cell therapy costs in Turkey are relatively low. So, it's no wonder patients worldwide visit Turkey for NK-92 cell therapy procedures. However, price is not the only factor that affects choices. We suggest looking for hospitals that are safe and have NK-92 cell therapy reviews on Google. When people decide to seek medical help for NK-92 cell therapy, they will not only have low-cost procedures in Turkey but also the safest and best treatment.
At clinics or hospitals contracted with Healthy Türkiye, patients will receive the best NK-92 cell therapy from specialist doctors in Turkey at affordable rates. Healthy Türkiye teams provide medical attention, NK-92 cell therapy procedures, and high-quality treatment to patients at a minimum cost. When you contact Healthy Türkiye assistants, you can get free information about the cost of NK-92 cell therapy in Turkey and what this cost covers.
Why Is NK-92 Cell Therapy Cheaper in Turkey?
One of the main considerations before traveling abroad for NK-92 cell therapy is the cost-effectiveness of the whole process. Many patients think that when they add flight tickets and hotel expenses to their NK-92 cell therapy costs, it will become very expensive to travel, which is not true. Contrary to popular belief, round-trip flight tickets to Turkey for NK-92 cell therapy can be booked very affordably.
In this case, assuming you are staying in Turkey for your NK-92 cell therapy, your total travel expense of flight tickets and accommodation will be less than any other developed country, which is nothing compared to the amount that you are saving.
The question “Why is NK-92 cell therapy cheaper in Turkey?” is so common among patients or people simply curious about getting their medical treatment in Turkey. When it comes to NK-92 cell therapy prices in Turkey, there are 3 factors that allow cheaper prices:
The currency exchange is favorable for those looking for NK-92 cell therapy has a euro, dollar, or pound;
The lower cost of living and cheaper overall medical expenses, such as NK-92 cell therapy;
For NK-92 cell therapy, incentives are given by the Turkish Government to medical clinics working with international clients;
All these factors allow for cheaper NK-92 cell therapy prices, but let’s be clear, these prices are cheaper for people with strong currencies (as we said, euro, dollar, Canadian dollar, pound, etc).
Every year, thousands of patients from all over the world come to Turkey to get NK-92 cell therapy. The success of the healthcare system has increased in recent years, especially for NK-92 cell therapy. It’s easy to find well-educated and English-speaking medical professionals in Turkey for all kinds of medical treatment, such as NK-92 cell therapy.
Why Choose Turkey for NK-92 Cell Therapy?
Turkey is a common choice among international patients seeking advanced NK-92 cell therapy. Turkey’s health procedures are safe and effective operations with a high success rate, like NK-92 cell therapy. The increasing demand for high-quality NK-92 cell therapy at affordable prices has made Turkey a popular medical travel destination. In Turkey, NK-92 cell therapy is performed by highly experienced and trained doctors with the most advanced technology in the world. NK-92 cell therapy is done in Istanbul, Ankara, Antalya, and other major cities. The reasons for choosing NK-92 cell therapy in Turkey are as follows:
High-quality hospitals: Joint Commission International (JCI) accredited hospitals have dedicated NK-92 cell therapy units that are specially designed for patients. International and national strict protocols provide effective and successful NK-92 cell therapy for patients in Turkey.
Qualified experts: The expert teams include nurses and specialist doctors, working together to carry out NK-92 cell therapy according to the patient's needs. All the included doctors are highly experienced in performing NK-92 cell therapy.
Affordable price: The cost of NK-92 cell therapy in Turkey is affordable compared to Europe, the USA, the UK, Singapore, Australia, etc.
The high success rate: Highly experienced specialists, the best available technology, and stringently followed safety guidelines for post-operative care of the patient, resulting in a high success rate for NK-92 cell therapy in Turkey.
Is NK-92 Cell Therapy Safe in Turkey?
Did you know Turkey is one of the most visited destinations for NK-92 cell therapy in the world? It is ranked as one of the most visited tourist destinations for NK-92 cell therapy. Over the years, it has also come to be a very popular medical tourism destination too with many tourists coming in for NK-92 cell therapy. There are so many reasons why Turkey stands out as a leading destination for NK-92 cell therapy. Because Turkey is both safe and easy to travel to too with a regional airport hub and flight connections to pretty much everywhere, it is preferred for NK-92 cell therapy.
The best hospitals in Turkey have experienced medical staff and specialists who have performed thousands of medical services, such as NK-92 cell therapy. All procedures and coordination related to NK-92 cell therapy are controlled by the Ministry of Health in accordance with the law. Over many years, the greatest progress in medicine has been observed in the field of NK-92 cell therapy. Turkey is known among foreign patients for its great opportunities in the area of NK-92 cell therapy.
To emphasize, besides the price itself, the key factor in selecting a destination for NK-92 cell therapy is certainly the standard of medical services, the hospital staff’s high expertise, hospitality, and the safety of the country.
All-Inclusive Package for NK-92 Cell Therapy in Turkey
Healthy Türkiye offers all-inclusive packages for NK-92 cell therapy in Turkey at much lower prices. Extremely professional and experienced doctors and technicians carry out the high-quality NK-92 cell therapy. The cost of NK-92 cell therapy in European countries can be quite expensive, especially in the UK. Healthy Türkiye provides cheap all-inclusive packages for long and short stays of NK-92 cell therapy in Turkey. Because of many factors, we can provide you with many opportunities for your NK-92 cell therapy in Turkey.
The price of NK-92 cell therapy differs from other countries due to medical fees, staff labor prices, exchange rates, and market competition. You can save much more in NK-92 cell therapy compared to other countries in Turkey. When you purchase the NK-92 cell therapy all-inclusive package with Healthy Türkiye, our healthcare team will present of hotels for you to choose from. In NK-92 cell therapy travel, you will have the price of your stay included in the all-inclusive package cost.
In Turkey, when you purchase NK-92 cell therapy all-inclusive packages through Healthy Türkiye, you will always receive VIP transfers. These are provided by Healthy Türkiye, which is contracted with highly qualified hospitals for NK-92 cell therapy in Turkey. Healthy Türkiye teams will organize everything about NK-92 cell therapy for you and have you picked up from the airport and safely brought to your accommodation. Once settled in the hotel, you will be transferred to and from the clinic or hospital for NK-92 cell therapy. After your NK-92 cell therapy has been successfully completed, the transfer team will return you to the airport in time for your flight home. In Turkey, all packages of NK-92 cell therapy can be arranged upon request, which relaxes the minds of our patients.
Frequently Asked Questions
NK-92 Cell Therapy in Turkey uses off-the-shelf NK cells that target cancer cells without causing graft-versus-host disease (GvHD), offering a safer and more accessible alternative to traditional therapies.
The treatment involves multiple infusion cycles, usually over a few weeks, with each infusion lasting around 30-60 minutes. The total duration depends on the patient's response and treatment plan.
NK-92 Cell Therapy in Turkey is typically recommended for patients with relapsed or refractory cancer who have not responded to conventional treatments and meet the eligibility criteria for the therapy.
While NK-92 Cell Therapy in Turkey is generally well-tolerated, some patients may experience mild side effects, such as fever, chills, or fatigue, which usually resolve within a few hours.
Yes, NK-92 Cell Therapy in Turkey is often used in combination with other treatments, such as low-dose chemotherapy or radiotherapy, to enhance its effectiveness against certain cancers.
To determine if NK-92 Cell Therapy in Turkey is suitable for you, it's important to consult with a specialist who will evaluate your medical history, cancer type, and treatment goals.
